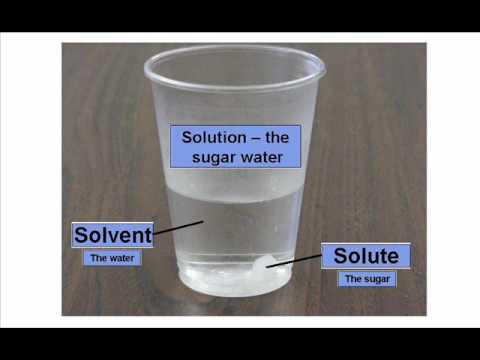
Today in class we discussed solutions. Solutions are homo- geneous mixtures comprised of solutes dispersed in a solvent. Water is the universal solvent, but not the only solvent. For example, a marshmallow is a solid (sugar) solute dispersed in a gaseous solvent (air).
Solubility is how well something dissolves. Some things are very soluble, and some are insoluble (do not dissolve).
Solutions are said to be saturated if they are holding all the solute that they can. When the solute starts to build up on the bottom, you know a solution is definitely saturated (like the dark blue solution on the right). Solutions are unsaturated if they can dissolve more solute (like the two light blue solutions on the left).
Solutions can be super-saturated if they are heated because they can hold more solute than normal. Even if you cool these solutions back down, they will still hold this additional solute in solution. Sweet Tea and all candies are made by first making super-saturated solutions and then cooling them.
For an excellent website about all of these topics and others regarding solutions that have and will be covered in this unit - check out this useful website.









No comments:
Post a Comment